Atom Number And Mass Number
Atom Number And Mass Number. Atoms of different elements usually have different mass numbers , but they can be the same. Mathematically, the atomic number is defined as.
Tady Question 29f82 Example
The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity. 04/02/2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. 20/09/2021 · atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. Atoms of different elements usually have different mass numbers , but they can be the same. This approximation of mass can be used to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number.20/07/2018 · atomic number and mass number.
This approximation of mass can be used to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number. The mass number of an atom is the total number of protons and neutrons in its nucleus. The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of … Mathematically, the atomic number is defined as. The mass number of an atom is its total number of protons and neutrons.

The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity. The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of … Atoms of different elements usually have different mass numbers , but they can be the same. Atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The mass number of an atom is the total number of protons and neutrons in its nucleus. 20/07/2018 · atomic number and mass number. Mathematically, the atomic number is defined as. 20/09/2021 · atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. 04/02/2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. An element's mass number (a) is the sum of the number of protons and the number of neutrons. The small contribution of mass from electrons is disregarded in calculating the mass number. Atoms of different elements usually have different mass numbers , but they can be the same.

The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity. Protons and neutrons both weigh … Atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Atoms of different elements usually have different mass numbers, but they can be the same. 04/02/2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. 20/09/2021 · atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. The small contribution of mass from electrons is disregarded in calculating the mass number. The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of … The mass number of an atom is its total number of protons and neutrons. Atoms of different elements usually have different mass numbers , but they can be the same. 20/07/2018 · atomic number and mass number. Atoms of different elements usually have different mass numbers, but they can be the same.

20/09/2021 · atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. 20/09/2021 · atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. Mathematically, the atomic number is defined as. The sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom. 04/02/2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. This approximation of mass can be used to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number. The mass number of an atom is the total number of protons and neutrons in its nucleus. Atoms of different elements usually have different mass numbers, but they can be the same. The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of … The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity... The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity.

The mass number of an atom is the total number of protons and neutrons in its nucleus. The small contribution of mass from electrons is disregarded in calculating the mass number. 04/02/2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of … An element's mass number (a) is the sum of the number of protons and the number of neutrons. Atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Atoms of different elements usually have different mass numbers , but they can be the same. The mass number of an atom is its total number of protons and neutrons. The mass number of an atom is the total number of protons and neutrons in its nucleus.

20/09/2021 · atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. An element's mass number (a) is the sum of the number of protons and the number of neutrons. 20/07/2018 · atomic number and mass number.

Mathematically, the atomic number is defined as. The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity.. An element's mass number (a) is the sum of the number of protons and the number of neutrons.

Atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons).. Atoms of different elements usually have different mass numbers, but they can be the same. The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of … This approximation of mass can be used to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number.

Atoms of different elements usually have different mass numbers , but they can be the same. The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of … Atoms of different elements usually have different mass numbers, but they can be the same. Protons and neutrons both weigh …

Mathematically, the atomic number is defined as... An element's mass number (a) is the sum of the number of protons and the number of neutrons. This approximation of mass can be used to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number. Protons and neutrons both weigh … The small contribution of mass from electrons is disregarded in calculating the mass number. 20/07/2018 · atomic number and mass number. Atoms of different elements usually have different mass numbers , but they can be the same.

04/02/2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. Protons and neutrons both weigh … Atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). An element's mass number (a) is the sum of the number of protons and the number of neutrons. The mass number of an atom is the total number of protons and neutrons in its nucleus. Atoms of different elements usually have different mass numbers, but they can be the same. 20/07/2018 · atomic number and mass number. Mathematically, the atomic number is defined as. This approximation of mass can be used to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number. Atoms of different elements usually have different mass numbers , but they can be the same. The mass number of an atom is its total number of protons and neutrons.. An element's mass number (a) is the sum of the number of protons and the number of neutrons.

Protons and neutrons both weigh … The sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom. Atoms of different elements usually have different mass numbers , but they can be the same. The small contribution of mass from electrons is disregarded in calculating the mass number. This approximation of mass can be used to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number. 04/02/2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons.. The mass number of an atom is the total number of protons and neutrons in its nucleus.
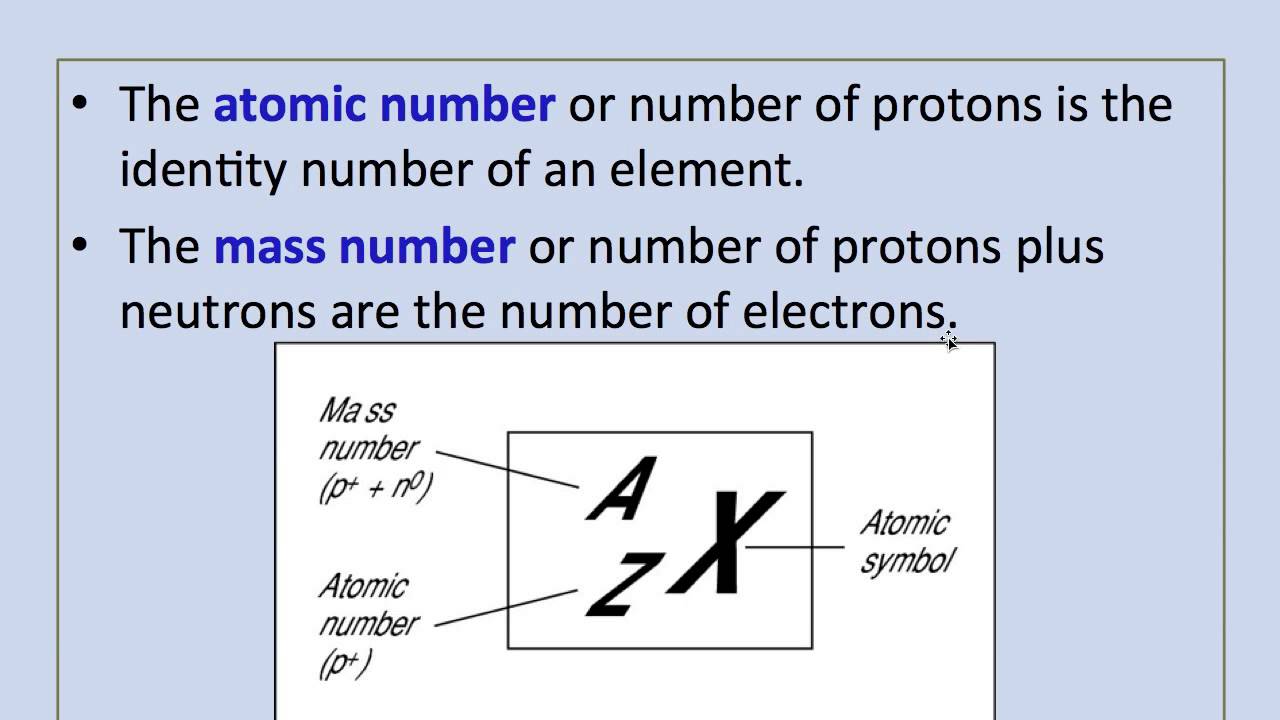
The small contribution of mass from electrons is disregarded in calculating the mass number. The mass number of an atom is the total number of protons and neutrons in its nucleus... The sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom.

Protons and neutrons both weigh ….. The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of … Atoms of different elements usually have different mass numbers, but they can be the same. This approximation of mass can be used to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number... 20/09/2021 · atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons.
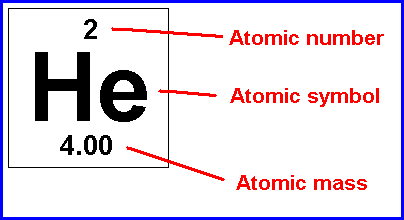
Atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Protons and neutrons both weigh … The mass number of an atom is the total number of protons and neutrons in its nucleus. Atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Mathematically, the atomic number is defined as. The mass number of an atom is its total number of protons and neutrons. An element's mass number (a) is the sum of the number of protons and the number of neutrons. Atoms of different elements usually have different mass numbers, but they can be the same.. 20/07/2018 · atomic number and mass number.

The sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom.. Atoms of different elements usually have different mass numbers , but they can be the same. The small contribution of mass from electrons is disregarded in calculating the mass number. 20/09/2021 · atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. The small contribution of mass from electrons is disregarded in calculating the mass number.

20/09/2021 · atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity. This approximation of mass can be used to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number. 04/02/2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. Protons and neutrons both weigh … The sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom.

The small contribution of mass from electrons is disregarded in calculating the mass number. An element's mass number (a) is the sum of the number of protons and the number of neutrons. Atoms of different elements usually have different mass numbers, but they can be the same. 04/02/2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. This approximation of mass can be used to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number. 20/09/2021 · atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of … Mathematically, the atomic number is defined as. The mass number of an atom is its total number of protons and neutrons. 20/07/2018 · atomic number and mass number. Atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The mass number of an atom is its total number of protons and neutrons.

04/02/2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons... The mass number of an atom is the total number of protons and neutrons in its nucleus. 20/09/2021 · atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. Atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). This approximation of mass can be used to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number. 04/02/2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. Atoms of different elements usually have different mass numbers , but they can be the same. Mathematically, the atomic number is defined as. The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity.. Atoms of different elements usually have different mass numbers , but they can be the same.

The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of … Atoms of different elements usually have different mass numbers , but they can be the same.

This approximation of mass can be used to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number. 20/09/2021 · atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. Mathematically, the atomic number is defined as. The sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom. 20/07/2018 · atomic number and mass number.. Protons and neutrons both weigh …

The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity. Atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons)... Atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons).

Atoms of different elements usually have different mass numbers , but they can be the same. This approximation of mass can be used to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number. An element's mass number (a) is the sum of the number of protons and the number of neutrons. 04/02/2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity.. The mass number of an atom is its total number of protons and neutrons.

The mass number of an atom is the total number of protons and neutrons in its nucleus. The small contribution of mass from electrons is disregarded in calculating the mass number. Protons and neutrons both weigh … Atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons).

An element's mass number (a) is the sum of the number of protons and the number of neutrons... Atoms of different elements usually have different mass numbers , but they can be the same.

The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of … Mathematically, the atomic number is defined as. 04/02/2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. The mass number of an atom is the total number of protons and neutrons in its nucleus. This approximation of mass can be used to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number.. The small contribution of mass from electrons is disregarded in calculating the mass number.

An element's mass number (a) is the sum of the number of protons and the number of neutrons. 20/07/2018 · atomic number and mass number. An element's mass number (a) is the sum of the number of protons and the number of neutrons. The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of … The mass number of an atom is the total number of protons and neutrons in its nucleus.. An element's mass number (a) is the sum of the number of protons and the number of neutrons.

The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity... 20/07/2018 · atomic number and mass number.

Atoms of different elements usually have different mass numbers , but they can be the same... The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity... Atoms of different elements usually have different mass numbers, but they can be the same.

20/09/2021 · atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons.. 04/02/2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. The mass number of an atom is its total number of protons and neutrons... Atoms of different elements usually have different mass numbers , but they can be the same.
20/07/2018 · atomic number and mass number. 04/02/2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. Atoms of different elements usually have different mass numbers , but they can be the same. The mass number of an atom is its total number of protons and neutrons. The sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom. The mass number of an atom is the total number of protons and neutrons in its nucleus. Mathematically, the atomic number is defined as.

The mass number of an atom is its total number of protons and neutrons. The mass number of an atom is its total number of protons and neutrons. The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity. Protons and neutrons both weigh … The small contribution of mass from electrons is disregarded in calculating the mass number. Atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons).. The mass number of an atom is its total number of protons and neutrons.

Protons and neutrons both weigh …. 04/02/2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. The sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom. The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity. 20/09/2021 · atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of … Protons and neutrons both weigh … This approximation of mass can be used to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number. Atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Atoms of different elements usually have different mass numbers, but they can be the same. An element's mass number (a) is the sum of the number of protons and the number of neutrons.
Mathematically, the atomic number is defined as.. An element's mass number (a) is the sum of the number of protons and the number of neutrons. Atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). 20/09/2021 · atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. The mass number of an atom is the total number of protons and neutrons in its nucleus. The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity.. The small contribution of mass from electrons is disregarded in calculating the mass number.

Atoms of different elements usually have different mass numbers , but they can be the same.. Atoms of different elements usually have different mass numbers , but they can be the same. The mass number of an atom is its total number of protons and neutrons. The sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom. Atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The mass number of an atom is the total number of protons and neutrons in its nucleus. Atoms of different elements usually have different mass numbers, but they can be the same. Mathematically, the atomic number is defined as. Protons and neutrons both weigh …

This approximation of mass can be used to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number... The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity. 20/09/2021 · atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons.. 04/02/2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons.

The mass number of an atom is the total number of protons and neutrons in its nucleus. The small contribution of mass from electrons is disregarded in calculating the mass number. Atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). 20/07/2018 · atomic number and mass number. Protons and neutrons both weigh … The sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom. The small contribution of mass from electrons is disregarded in calculating the mass number.

The sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom.. 20/09/2021 · atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. An element's mass number (a) is the sum of the number of protons and the number of neutrons. The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of … Protons and neutrons both weigh … Mathematically, the atomic number is defined as. The mass number of an atom is its total number of protons and neutrons. The mass number of an atom is the total number of protons and neutrons in its nucleus. The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity.. The sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom.

The sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom. The small contribution of mass from electrons is disregarded in calculating the mass number. Atoms of different elements usually have different mass numbers , but they can be the same. The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of … Mathematically, the atomic number is defined as. Atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity. 04/02/2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. This approximation of mass can be used to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number. An element's mass number (a) is the sum of the number of protons and the number of neutrons.. An element's mass number (a) is the sum of the number of protons and the number of neutrons.

The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity. The small contribution of mass from electrons is disregarded in calculating the mass number. Protons and neutrons both weigh … The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of … Mathematically, the atomic number is defined as. Atoms of different elements usually have different mass numbers , but they can be the same.. 20/09/2021 · atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons.

The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of … 04/02/2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. 20/09/2021 · atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. The mass number of an atom is the total number of protons and neutrons in its nucleus. The small contribution of mass from electrons is disregarded in calculating the mass number. The sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom. This approximation of mass can be used to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number. Protons and neutrons both weigh … The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of … 20/07/2018 · atomic number and mass number.. Atoms of different elements usually have different mass numbers , but they can be the same.

An element's mass number (a) is the sum of the number of protons and the number of neutrons. This approximation of mass can be used to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number. Atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). 04/02/2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. The sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom. Atoms of different elements usually have different mass numbers , but they can be the same. The small contribution of mass from electrons is disregarded in calculating the mass number. 20/09/2021 · atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. Atoms of different elements usually have different mass numbers , but they can be the same.

The sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom. This approximation of mass can be used to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number. The small contribution of mass from electrons is disregarded in calculating the mass number. Protons and neutrons both weigh … 20/07/2018 · atomic number and mass number. Mathematically, the atomic number is defined as. The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of … The mass number of an atom is its total number of protons and neutrons. The sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom. Atoms of different elements usually have different mass numbers, but they can be the same.. 04/02/2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons.

The sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom. An element's mass number (a) is the sum of the number of protons and the number of neutrons. Protons and neutrons both weigh … The sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom. Atoms of different elements usually have different mass numbers, but they can be the same. The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of … The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity. 20/09/2021 · atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. Protons and neutrons both weigh …

The small contribution of mass from electrons is disregarded in calculating the mass number... Mathematically, the atomic number is defined as. 20/09/2021 · atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons.. An element's mass number (a) is the sum of the number of protons and the number of neutrons.

Atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Protons and neutrons both weigh … The small contribution of mass from electrons is disregarded in calculating the mass number. The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity. Atoms of different elements usually have different mass numbers, but they can be the same. 20/07/2018 · atomic number and mass number. The mass number of an atom is the total number of protons and neutrons in its nucleus. 20/09/2021 · atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. 04/02/2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. The mass number of an atom is its total number of protons and neutrons. The sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom. Atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons).

This approximation of mass can be used to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number... The mass number of an atom is its total number of protons and neutrons.. 20/07/2018 · atomic number and mass number.

The mass number of an atom is the total number of protons and neutrons in its nucleus... The mass number of an atom is its total number of protons and neutrons. The small contribution of mass from electrons is disregarded in calculating the mass number. Atoms of different elements usually have different mass numbers, but they can be the same. 04/02/2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. An element's mass number (a) is the sum of the number of protons and the number of neutrons. The mass number of an atom is the total number of protons and neutrons in its nucleus. 20/09/2021 · atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. The sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom. Mathematically, the atomic number is defined as. Atoms of different elements usually have different mass numbers , but they can be the same. 04/02/2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons.

The mass number of an atom is its total number of protons and neutrons. 04/02/2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. Atoms of different elements usually have different mass numbers , but they can be the same. Protons and neutrons both weigh … The mass number of an atom is the total number of protons and neutrons in its nucleus.. This approximation of mass can be used to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number.

04/02/2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity. The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of … The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of …

Atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). . An element's mass number (a) is the sum of the number of protons and the number of neutrons.

Atoms of different elements usually have different mass numbers, but they can be the same... The mass number of an atom is the total number of protons and neutrons in its nucleus.. 04/02/2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons.

This approximation of mass can be used to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number. 04/02/2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. The mass number of an atom is its total number of protons and neutrons. 20/07/2018 · atomic number and mass number... Atoms of different elements usually have different mass numbers, but they can be the same.

Mathematically, the atomic number is defined as. The mass number of an atom is the total number of protons and neutrons in its nucleus. The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of …

The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity... 04/02/2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons... 20/07/2018 · atomic number and mass number.

20/09/2021 · atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons... Protons and neutrons both weigh … Mathematically, the atomic number is defined as. 20/09/2021 · atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. 04/02/2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. The mass number of an atom is its total number of protons and neutrons.

This approximation of mass can be used to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number. This approximation of mass can be used to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number. Atoms of different elements usually have different mass numbers, but they can be the same. An element's mass number (a) is the sum of the number of protons and the number of neutrons. Protons and neutrons both weigh ….. An element's mass number (a) is the sum of the number of protons and the number of neutrons.
