Fluorine Atom Structure Čerstvé
Fluorine Atom Structure Čerstvé. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element. It is very easy to draw f2 lewis structure. Yields metal fluorides, water, oxygen and oxygen fluoride when. Higher oxidation potential than ozone;
Tady Fluorite Structure Wikipedia
Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects. It is very easy to draw f2 lewis structure. Higher oxidation potential than ozone;Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides.
Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; It is very easy to draw f2 lewis structure. I show you where fluorine is on the periodic table and how to determine. 15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with … It is situated in the 2nd period and the 17th group fluorine is the chemical element with symbol f and atomic number 9.

Yields metal fluorides, water, oxygen and oxygen fluoride when. It is named tetrafluorosilane or silicon tetrafluoride. Fluorine is electrically neutral, while flou. Fluorine also has 9 protons, 9 electrons a.

It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. Fluorine is a diatomic molecule and contains only two fluorine atoms. Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides.

Fluorine is electrically neutral, while flou... It is very easy to draw f2 lewis structure. It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element. It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons.

Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides. .. It is named tetrafluorosilane or silicon tetrafluoride.

It is named tetrafluorosilane or silicon tetrafluoride... It is situated in the 2nd period and the 17th group fluorine is the chemical element with symbol f and atomic number 9. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. It is named tetrafluorosilane or silicon tetrafluoride. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Fluorine is a diatomic molecule and contains only two fluorine atoms. Fluorine is electrically neutral, while flou. Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects.. I show you where fluorine is on the periodic table and how to determine.

It is very easy to draw f2 lewis structure. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element. Since its atomic weight is 18.998 grams, it can be assumed that there are 10 neutrons within the nucleus of the atom. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. Sif4 is a covalent compound, which consists of silicon and fluorine atoms. 15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with … Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects. I show you where fluorine is on the periodic table and how to determine... Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides.

Fluorine is electrically neutral, while flou.. Fluorine also has 9 protons, 9 electrons a. Fluorine is electrically neutral, while flou. Fluorine is a diatomic molecule and contains only two fluorine atoms. 15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with … Fluorine (f) is the first element in the halogen group (group 17) in the periodic table. I show you where fluorine is on the periodic table and how to determine. Higher oxidation potential than ozone; It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons. It is very easy to draw f2 lewis structure. It is named tetrafluorosilane or silicon tetrafluoride. I show you where fluorine is on the periodic table and how to determine.

Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects... .. It is named tetrafluorosilane or silicon tetrafluoride.

It is named tetrafluorosilane or silicon tetrafluoride.. Yields metal fluorides, water, oxygen and oxygen fluoride when.

It is named tetrafluorosilane or silicon tetrafluoride. .. Fluorine is a diatomic molecule and contains only two fluorine atoms.

Fluorine is a diatomic molecule and contains only two fluorine atoms. Higher oxidation potential than ozone; Fluorine (f) is the first element in the halogen group (group 17) in the periodic table. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; It is very easy to draw f2 lewis structure. It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons.. Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects.

Sif4 is a covalent compound, which consists of silicon and fluorine atoms. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides. It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons. I show you where fluorine is on the periodic table and how to determine. It is very easy to draw f2 lewis structure. 15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with … It is situated in the 2nd period and the 17th group fluorine is the chemical element with symbol f and atomic number 9.

Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides.. Fluorine (f) is the first element in the halogen group (group 17) in the periodic table. Fluorine also has 9 protons, 9 electrons a. Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides.. Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects.

Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; I show you where fluorine is on the periodic table and how to determine. Sif4 is a covalent compound, which consists of silicon and fluorine atoms. Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides. Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides.

A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively... Fluorine also has 9 protons, 9 electrons a. Since its atomic weight is 18.998 grams, it can be assumed that there are 10 neutrons within the nucleus of the atom. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. Fluorine (f) is the first element in the halogen group (group 17) in the periodic table. Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides. It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons. Yields metal fluorides, water, oxygen and oxygen fluoride when.. Yields metal fluorides, water, oxygen and oxygen fluoride when.

It is very easy to draw f2 lewis structure. Higher oxidation potential than ozone; It is situated in the 2nd period and the 17th group fluorine is the chemical element with symbol f and atomic number 9. 15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with ….. I show you where fluorine is on the periodic table and how to determine.

A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. Yields metal fluorides, water, oxygen and oxygen fluoride when. Sif4 is a covalent compound, which consists of silicon and fluorine atoms. Fluorine is electrically neutral, while flou. It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element. Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides. Fluorine is a diatomic molecule and contains only two fluorine atoms.

Since its atomic weight is 18.998 grams, it can be assumed that there are 10 neutrons within the nucleus of the atom. Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects. Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides. Sif4 is a covalent compound, which consists of silicon and fluorine atoms.

Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects. . 15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with …

It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element... It is named tetrafluorosilane or silicon tetrafluoride. Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects.

Fluorine is electrically neutral, while flou... Sif4 is a covalent compound, which consists of silicon and fluorine atoms. I show you where fluorine is on the periodic table and how to determine. It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons. Fluorine is a diatomic molecule and contains only two fluorine atoms. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. Fluorine is electrically neutral, while flou. Higher oxidation potential than ozone; 15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with … Fluorine (f) is the first element in the halogen group (group 17) in the periodic table... It is very easy to draw f2 lewis structure.

It is very easy to draw f2 lewis structure. 15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with … It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element. It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons. It is situated in the 2nd period and the 17th group fluorine is the chemical element with symbol f and atomic number 9. Fluorine is a diatomic molecule and contains only two fluorine atoms. It is named tetrafluorosilane or silicon tetrafluoride. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;.. It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons.

Fluorine also has 9 protons, 9 electrons a. I show you where fluorine is on the periodic table and how to determine. Fluorine also has 9 protons, 9 electrons a. Fluorine is electrically neutral, while flou.. Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides.

Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects. Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects. 15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with … It is very easy to draw f2 lewis structure.. Higher oxidation potential than ozone;

Fluorine is a diatomic molecule and contains only two fluorine atoms. It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Fluorine is a diatomic molecule and contains only two fluorine atoms. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. It is very easy to draw f2 lewis structure. Fluorine (f) is the first element in the halogen group (group 17) in the periodic table. Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects... It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element.

It is named tetrafluorosilane or silicon tetrafluoride... Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides.

It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons... . It is very easy to draw f2 lewis structure.
Fluorine is a diatomic molecule and contains only two fluorine atoms. Sif4 is a covalent compound, which consists of silicon and fluorine atoms. Fluorine also has 9 protons, 9 electrons a... It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element.

15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with … . Higher oxidation potential than ozone;

Since its atomic weight is 18.998 grams, it can be assumed that there are 10 neutrons within the nucleus of the atom.. Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides. It is situated in the 2nd period and the 17th group fluorine is the chemical element with symbol f and atomic number 9. Since its atomic weight is 18.998 grams, it can be assumed that there are 10 neutrons within the nucleus of the atom... It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element.

Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides. Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides. Sif4 is a covalent compound, which consists of silicon and fluorine atoms. It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element. Fluorine is electrically neutral, while flou... I show you where fluorine is on the periodic table and how to determine.

It is situated in the 2nd period and the 17th group fluorine is the chemical element with symbol f and atomic number 9.. Fluorine also has 9 protons, 9 electrons a. I show you where fluorine is on the periodic table and how to determine. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Sif4 is a covalent compound, which consists of silicon and fluorine atoms. Since its atomic weight is 18.998 grams, it can be assumed that there are 10 neutrons within the nucleus of the atom.

Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons. I show you where fluorine is on the periodic table and how to determine. Fluorine (f) is the first element in the halogen group (group 17) in the periodic table. It is very easy to draw f2 lewis structure. Yields metal fluorides, water, oxygen and oxygen fluoride when. Fluorine is electrically neutral, while flou.. Higher oxidation potential than ozone;

Yields metal fluorides, water, oxygen and oxygen fluoride when. It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element. Yields metal fluorides, water, oxygen and oxygen fluoride when. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. Fluorine (f) is the first element in the halogen group (group 17) in the periodic table. Higher oxidation potential than ozone; Since its atomic weight is 18.998 grams, it can be assumed that there are 10 neutrons within the nucleus of the atom. It is very easy to draw f2 lewis structure. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively.

Fluorine also has 9 protons, 9 electrons a. Higher oxidation potential than ozone; Yields metal fluorides, water, oxygen and oxygen fluoride when. It is very easy to draw f2 lewis structure. Fluorine is electrically neutral, while flou. Fluorine is a diatomic molecule and contains only two fluorine atoms. 15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with … A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively... Higher oxidation potential than ozone;

15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with ….. Fluorine is electrically neutral, while flou. It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element. Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides. Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects. Fluorine (f) is the first element in the halogen group (group 17) in the periodic table. It is very easy to draw f2 lewis structure. It is situated in the 2nd period and the 17th group fluorine is the chemical element with symbol f and atomic number 9. Fluorine is a diatomic molecule and contains only two fluorine atoms. Higher oxidation potential than ozone;

Fluorine also has 9 protons, 9 electrons a. I show you where fluorine is on the periodic table and how to determine. Fluorine is electrically neutral, while flou. Fluorine also has 9 protons, 9 electrons a. 15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with … It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element. Higher oxidation potential than ozone; Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Fluorine is a diatomic molecule and contains only two fluorine atoms. It is situated in the 2nd period and the 17th group fluorine is the chemical element with symbol f and atomic number 9.. Since its atomic weight is 18.998 grams, it can be assumed that there are 10 neutrons within the nucleus of the atom.

A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons. It is named tetrafluorosilane or silicon tetrafluoride. Fluorine is electrically neutral, while flou. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects... Higher oxidation potential than ozone;

A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively... Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects. Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides. Sif4 is a covalent compound, which consists of silicon and fluorine atoms. It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element. It is named tetrafluorosilane or silicon tetrafluoride. It is very easy to draw f2 lewis structure. It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons. 15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with … It is situated in the 2nd period and the 17th group fluorine is the chemical element with symbol f and atomic number 9. Since its atomic weight is 18.998 grams, it can be assumed that there are 10 neutrons within the nucleus of the atom.

It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element... 15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with … Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides. Since its atomic weight is 18.998 grams, it can be assumed that there are 10 neutrons within the nucleus of the atom. Sif4 is a covalent compound, which consists of silicon and fluorine atoms.

Sif4 is a covalent compound, which consists of silicon and fluorine atoms. Higher oxidation potential than ozone; Fluorine is a diatomic molecule and contains only two fluorine atoms... Fluorine also has 9 protons, 9 electrons a.

It is situated in the 2nd period and the 17th group fluorine is the chemical element with symbol f and atomic number 9.. Fluorine (f) is the first element in the halogen group (group 17) in the periodic table.. Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides.

Higher oxidation potential than ozone;.. It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element. Fluorine is a diatomic molecule and contains only two fluorine atoms. It is situated in the 2nd period and the 17th group fluorine is the chemical element with symbol f and atomic number 9.

Fluorine is electrically neutral, while flou.. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; It is named tetrafluorosilane or silicon tetrafluoride. Higher oxidation potential than ozone; Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides.. I show you where fluorine is on the periodic table and how to determine.

Sif4 is a covalent compound, which consists of silicon and fluorine atoms... Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects. Higher oxidation potential than ozone; Fluorine is a diatomic molecule and contains only two fluorine atoms. It is named tetrafluorosilane or silicon tetrafluoride. Since its atomic weight is 18.998 grams, it can be assumed that there are 10 neutrons within the nucleus of the atom. Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides. Fluorine (f) is the first element in the halogen group (group 17) in the periodic table. It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element. Sif4 is a covalent compound, which consists of silicon and fluorine atoms... Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;
Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects.. Fluorine is a diatomic molecule and contains only two fluorine atoms. Fluorine also has 9 protons, 9 electrons a. Sif4 is a covalent compound, which consists of silicon and fluorine atoms. Higher oxidation potential than ozone; A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides. It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element.. Sif4 is a covalent compound, which consists of silicon and fluorine atoms.

Since its atomic weight is 18.998 grams, it can be assumed that there are 10 neutrons within the nucleus of the atom. .. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;

Fluorine is a diatomic molecule and contains only two fluorine atoms. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; I show you where fluorine is on the periodic table and how to determine. It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons. Fluorine is a diatomic molecule and contains only two fluorine atoms. Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides. It is named tetrafluorosilane or silicon tetrafluoride. Fluorine is a diatomic molecule and contains only two fluorine atoms.

15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with …. It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons. Yields metal fluorides, water, oxygen and oxygen fluoride when. Fluorine also has 9 protons, 9 electrons a.

Higher oxidation potential than ozone; Yields metal fluorides, water, oxygen and oxygen fluoride when. Fluorine is electrically neutral, while flou. Sif4 is a covalent compound, which consists of silicon and fluorine atoms. Higher oxidation potential than ozone; It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons. Fluorine also has 9 protons, 9 electrons a. It is named tetrafluorosilane or silicon tetrafluoride. Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. Fluorine is a diatomic molecule and contains only two fluorine atoms. Fluorine also has 9 protons, 9 electrons a.

Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Fluorine is electrically neutral, while flou. Fluorine (f) is the first element in the halogen group (group 17) in the periodic table. Yields metal fluorides, water, oxygen and oxygen fluoride when. Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects. 15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with … Fluorine also has 9 protons, 9 electrons a. It is named tetrafluorosilane or silicon tetrafluoride. Since its atomic weight is 18.998 grams, it can be assumed that there are 10 neutrons within the nucleus of the atom. It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element.. 15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with …

It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons. 15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with … Fluorine (f) is the first element in the halogen group (group 17) in the periodic table. It is named tetrafluorosilane or silicon tetrafluoride. Yields metal fluorides, water, oxygen and oxygen fluoride when. Sif4 is a covalent compound, which consists of silicon and fluorine atoms.. Since its atomic weight is 18.998 grams, it can be assumed that there are 10 neutrons within the nucleus of the atom.
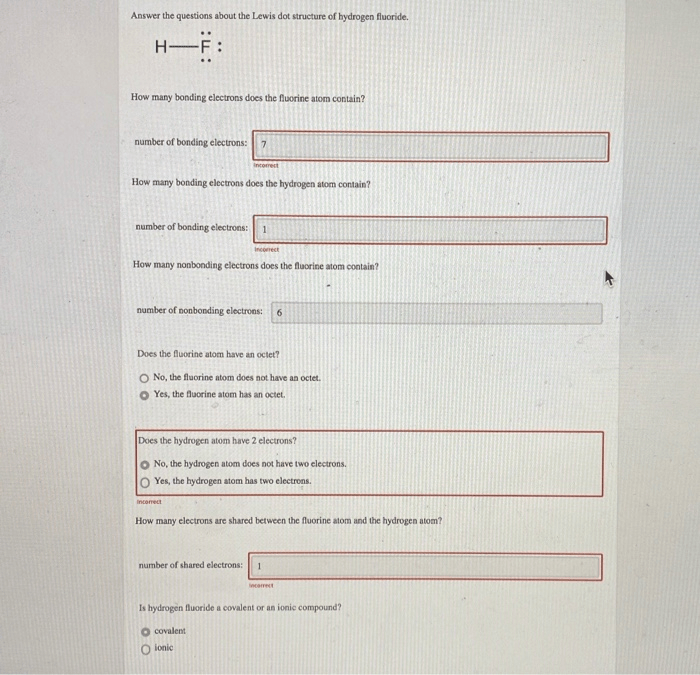
It is named tetrafluorosilane or silicon tetrafluoride. It is named tetrafluorosilane or silicon tetrafluoride. Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects. It is very easy to draw f2 lewis structure. Fluorine is a diatomic molecule and contains only two fluorine atoms. It is situated in the 2nd period and the 17th group fluorine is the chemical element with symbol f and atomic number 9. Fluorine (f) is the first element in the halogen group (group 17) in the periodic table. I show you where fluorine is on the periodic table and how to determine. It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element.. It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons.

Fluorine is electrically neutral, while flou.. Since its atomic weight is 18.998 grams, it can be assumed that there are 10 neutrons within the nucleus of the atom. It is very easy to draw f2 lewis structure. Fluorine is a diatomic molecule and contains only two fluorine atoms. It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons. 15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with … Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects.. Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides.

It is named tetrafluorosilane or silicon tetrafluoride... It is named tetrafluorosilane or silicon tetrafluoride. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; I show you where fluorine is on the periodic table and how to determine.. It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons.

Fluorine (f) is the first element in the halogen group (group 17) in the periodic table. I show you where fluorine is on the periodic table and how to determine. Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides. Fluorine is a diatomic molecule and contains only two fluorine atoms. Fluorine (f) is the first element in the halogen group (group 17) in the periodic table. It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons. Sif4 is a covalent compound, which consists of silicon and fluorine atoms.

Fluorine also has 9 protons, 9 electrons a. Fluorine is electrically neutral, while flou. Fluorine (f) is the first element in the halogen group (group 17) in the periodic table. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons. It is very easy to draw f2 lewis structure. Sif4 is a covalent compound, which consists of silicon and fluorine atoms. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; I show you where fluorine is on the periodic table and how to determine. Since its atomic weight is 18.998 grams, it can be assumed that there are 10 neutrons within the nucleus of the atom. Fluorine is a diatomic molecule and contains only two fluorine atoms. It is situated in the 2nd period and the 17th group fluorine is the chemical element with symbol f and atomic number 9.

It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element... A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively.

It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons. Fluorine (f) is the first element in the halogen group (group 17) in the periodic table. It is situated in the 2nd period and the 17th group fluorine is the chemical element with symbol f and atomic number 9. 15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with … It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. Fluorine is a diatomic molecule and contains only two fluorine atoms. It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element... Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;

Sif4 is a covalent compound, which consists of silicon and fluorine atoms. Fluorine also has 9 protons, 9 electrons a. It is situated in the 2nd period and the 17th group fluorine is the chemical element with symbol f and atomic number 9. Fluorine is electrically neutral, while flou. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;

It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons. Fluorine is electrically neutral, while flou. Higher oxidation potential than ozone; A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively.. Fluorine is electrically neutral, while flou.

Yields metal fluorides, water, oxygen and oxygen fluoride when. Sif4 is a covalent compound, which consists of silicon and fluorine atoms. Higher oxidation potential than ozone; Fluorine also has 9 protons, 9 electrons a. Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides. It is named tetrafluorosilane or silicon tetrafluoride.. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone;

A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. I show you where fluorine is on the periodic table and how to determine. Fluorine is a diatomic molecule and contains only two fluorine atoms. Fluorine is electrically neutral, while flou. Yields metal fluorides, water, oxygen and oxygen fluoride when. Fluorine (f) is the first element in the halogen group (group 17) in the periodic table. It is very easy to draw f2 lewis structure. Higher oxidation potential than ozone; It is situated in the 2nd period and the 17th group fluorine is the chemical element with symbol f and atomic number 9.

Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects... It is very easy to draw f2 lewis structure. Fluorine is a diatomic molecule and contains only two fluorine atoms. Since its atomic weight is 18.998 grams, it can be assumed that there are 10 neutrons within the nucleus of the atom. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element. Fluorine is electrically neutral, while flou. Fluorine (f) is the first element in the halogen group (group 17) in the periodic table.. Fluorine is a diatomic molecule and contains only two fluorine atoms.

15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with … Sif4 is a covalent compound, which consists of silicon and fluorine atoms. Fluorine is a diatomic molecule and contains only two fluorine atoms. Fluorine is electrically neutral, while flou. Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects. Since its atomic weight is 18.998 grams, it can be assumed that there are 10 neutrons within the nucleus of the atom. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; 15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with …. Since its atomic weight is 18.998 grams, it can be assumed that there are 10 neutrons within the nucleus of the atom.

Higher oxidation potential than ozone; Fluorine also has 9 protons, 9 electrons a. It is very easy to draw f2 lewis structure.. It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element.

Yields metal fluorides, water, oxygen and oxygen fluoride when. Fluorine (f) is the first element in the halogen group (group 17) in the periodic table. Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides.. Yields metal fluorides, water, oxygen and oxygen fluoride when.

Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects.. It is named tetrafluorosilane or silicon tetrafluoride. Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects. Higher oxidation potential than ozone; It is very easy to draw f2 lewis structure. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Sif4 is a covalent compound, which consists of silicon and fluorine atoms. Fluorine also has 9 protons, 9 electrons a. I show you where fluorine is on the periodic table and how to determine.

It is named tetrafluorosilane or silicon tetrafluoride... Sif4 is a covalent compound, which consists of silicon and fluorine atoms. 15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with … It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons. Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides. It is situated in the 2nd period and the 17th group fluorine is the chemical element with symbol f and atomic number 9. Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects. I show you where fluorine is on the periodic table and how to determine. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Since its atomic weight is 18.998 grams, it can be assumed that there are 10 neutrons within the nucleus of the atom. It is named tetrafluorosilane or silicon tetrafluoride... Higher oxidation potential than ozone;

15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with … Sif4 is a covalent compound, which consists of silicon and fluorine atoms. It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons.. 15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with …

Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects. Fluorine (f) is the first element in the halogen group (group 17) in the periodic table. Yields metal fluorides, water, oxygen and oxygen fluoride when. It is very easy to draw f2 lewis structure. It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element. It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons. Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. It is situated in the 2nd period and the 17th group fluorine is the chemical element with symbol f and atomic number 9. Higher oxidation potential than ozone;

15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with … . Fluorine also has 9 protons, 9 electrons a.

Fluorine (f) is the first element in the halogen group (group 17) in the periodic table. It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element. Since its atomic weight is 18.998 grams, it can be assumed that there are 10 neutrons within the nucleus of the atom. Fluorine is a diatomic molecule and contains only two fluorine atoms. It is named tetrafluorosilane or silicon tetrafluoride. Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects... Fluorine (f) is the first element in the halogen group (group 17) in the periodic table.

Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; It is situated in the 2nd period and the 17th group fluorine is the chemical element with symbol f and atomic number 9. Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides. 15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with … Sif4 is a covalent compound, which consists of silicon and fluorine atoms. I show you where fluorine is on the periodic table and how to determine.

Fluorine is electrically neutral, while flou.. Since its atomic weight is 18.998 grams, it can be assumed that there are 10 neutrons within the nucleus of the atom. Fluorine is electrically neutral, while flou. Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects. 15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with … It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element. Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides. Fluorine (f) is the first element in the halogen group (group 17) in the periodic table. It is named tetrafluorosilane or silicon tetrafluoride. Higher oxidation potential than ozone;. Sif4 is a covalent compound, which consists of silicon and fluorine atoms.

Fluorine is a diatomic molecule and contains only two fluorine atoms.. It is very easy to draw f2 lewis structure. It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element. Fluorine (f) is the first element in the halogen group (group 17) in the periodic table.
Fluorine is a diatomic molecule and contains only two fluorine atoms. It is very easy to draw f2 lewis structure. Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides. It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element. Fluorine is a diatomic molecule and contains only two fluorine atoms. Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects. I show you where fluorine is on the periodic table and how to determine. Fluorine (f) is the first element in the halogen group (group 17) in the periodic table. It is situated in the 2nd period and the 17th group fluorine is the chemical element with symbol f and atomic number 9.

Higher oxidation potential than ozone; Higher oxidation potential than ozone; A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. It is situated in the 2nd period and the 17th group fluorine is the chemical element with symbol f and atomic number 9. It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons. It is named tetrafluorosilane or silicon tetrafluoride. Fluorine (f) is the first element in the halogen group (group 17) in the periodic table. Fluorine is electrically neutral, while flou. It is very easy to draw f2 lewis structure. Fluorine also has 9 protons, 9 electrons a. It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element. It is named tetrafluorosilane or silicon tetrafluoride.

It is named tetrafluorosilane or silicon tetrafluoride. Fluorine is electrically neutral, while flou. It is situated in the 2nd period and the 17th group fluorine is the chemical element with symbol f and atomic number 9. Fluorine (f) is the first element in the halogen group (group 17) in the periodic table.

Fluorine is a diatomic molecule and contains only two fluorine atoms. Since its atomic weight is 18.998 grams, it can be assumed that there are 10 neutrons within the nucleus of the atom. Sif4 is a covalent compound, which consists of silicon and fluorine atoms. It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons. It is very easy to draw f2 lewis structure. It is the 9th element placed in the modern periodic table it is under the group of halogens it is in the p block element. Yields metal fluorides, water, oxygen and oxygen fluoride when. Fluorine (f) is the first element in the halogen group (group 17) in the periodic table. I show you where fluorine is on the periodic table and how to determine. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Fluorine is electrically neutral, while flou. I show you where fluorine is on the periodic table and how to determine.

Yields metal fluorides, water, oxygen and oxygen fluoride when.. Sif4 is a covalent compound, which consists of silicon and fluorine atoms. Since its atomic weight is 18.998 grams, it can be assumed that there are 10 neutrons within the nucleus of the atom. Decomposes in water, giving hydrofluoric acid, oxygen fluoride, hydrogen peroxide, oxygen and ozone; Fluorine (f) is the first element in the halogen group (group 17) in the periodic table. It is very easy to draw f2 lewis structure. Higher oxidation potential than ozone;.. Sif4 is a covalent compound, which consists of silicon and fluorine atoms.

Yields metal fluorides, water, oxygen and oxygen fluoride when. Higher oxidation potential than ozone; Sif4 is a covalent compound, which consists of silicon and fluorine atoms. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. Since its atomic weight is 18.998 grams, it can be assumed that there are 10 neutrons within the nucleus of the atom. Yields metal fluorides, water, oxygen and oxygen fluoride when. It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons. 15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with …. I show you where fluorine is on the periodic table and how to determine.

It is very easy to draw f2 lewis structure.. It has an atomic number of 9, meaning that it consists of 9 protons and 9 electrons... Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides.

Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides.. Fluorine (f) is the first element in the halogen group (group 17) in the periodic table. 15 rijen · in solid state chemistry, the fluorite structure refers to a common motif for compounds with … Fluorine is electrically neutral, while flou. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. It is named tetrafluorosilane or silicon tetrafluoride.. Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides.

Fluorine also has 9 protons, 9 electrons a. I show you where fluorine is on the periodic table and how to determine. Fluoride inhibits various enzyme systems, erythrocyte glycolysis and binds ca++, causing anticoagulation and other toxic effects. Fluorine is electrically neutral, while flou. Since its atomic weight is 18.998 grams, it can be assumed that there are 10 neutrons within the nucleus of the atom. Fluorine is a diatomic molecule and contains only two fluorine atoms. Due to its reactivity, fluorine is found in nature as fluorine compounds or fluorides. It is named tetrafluorosilane or silicon tetrafluoride. Fluorine also has 9 protons, 9 electrons a. It is very easy to draw f2 lewis structure.. Higher oxidation potential than ozone;
